________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Cosentyx (secukinumab) in Rheumatology
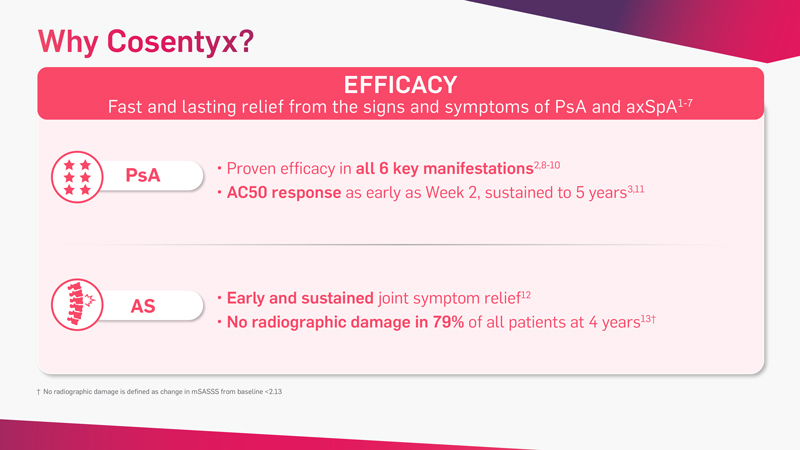
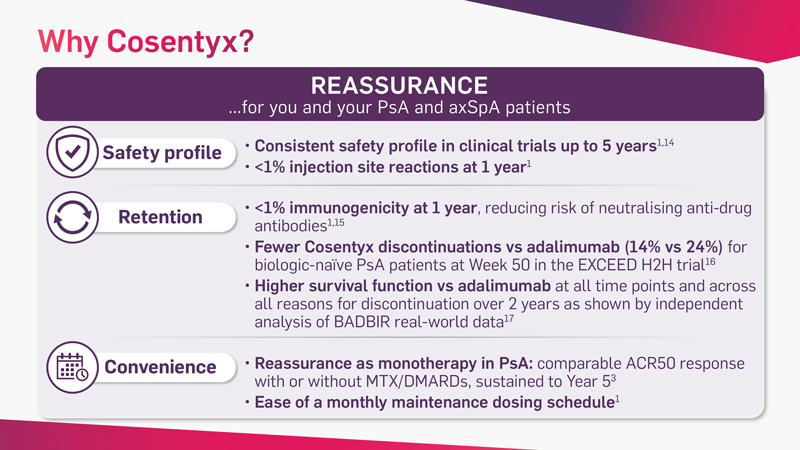
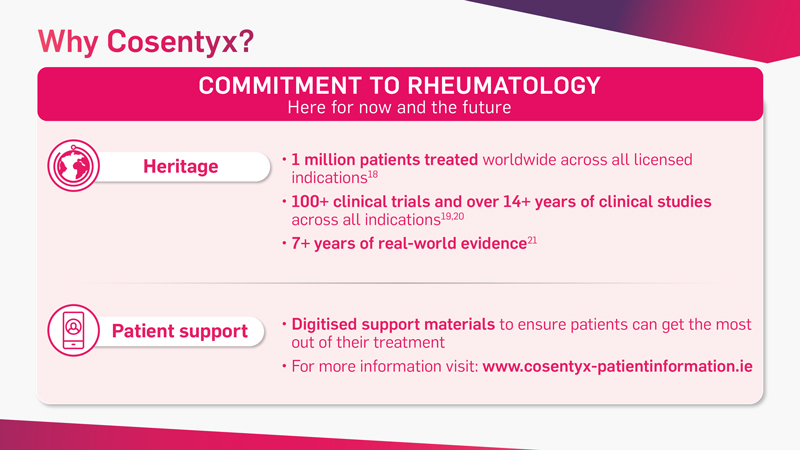
Cosentyx is the first and only fully human targeted IL-17A inhibitor that offers fast and lasting relief from the signs and symptoms of PsA* and axSpA†‡
*Patients with PsA receiving Cosentyx achieved significant improvement in ACR20 vs placebo at Week 24: 54% Cosentyx300 mg s.c.; 51% 150 mg s.c. vs 15% placebo P<0.0001.2 This improvement was sustained after 5 years of treatment.3
†Patients with AS receiving Cosentyx achieved significant improvement in ASAS20 vs placebo at Week 16: 61% Cosentyx 150 mg s.c. vs 28% placebo (P<0.001).5 This improvement was sustained after 5 years of treatment.6
‡Patients (NSAID-IR) with nr-axSpA receiving Cosentyx 150 mg s.c. vs placebo (N=555). Primary endpoint was met: ASAS40 at Week 16 and Week 52. ASAS40 vs placebo at Week 16: 42% Cosentyx 150 mg s.c. vs 29% placebo (P<0.05). This improvement was sustained after 1 year of treatment.7
δPatients with active AS receiving Cosentyx 150 mg (n=54 at Year 5).6
§Not limited to licensed indications.
**Across all licensed indications.
ACR, American College of Rheumatology; AS, ankylosing spondylitis; ASAS, Assessment in SpondyloArthritis international Society; axSpA, axial spondyloarthritis; BAD, British Association of Dermatologists; DMARD, disease-modifying antirheumatic drug; EULAR, European League Against Rheumatism IL, interleukin; ISR, injection site reaction; MRI, magnetic resonance imaging; nr-axSpA, non-radiographic axial spondyloarthritis; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; PsO, plaque psoriasis; s.c. subcutaneous; TB, tuberculosis.
- Cosentyx SmPC Available at www.medicines.ie last accessed November 2024
- McInnes IB et al. Lancet. 2015;386:1137–46.
- McInnes IB et al. Lancet Rheumatol. 2020;2:e227–35.
- Rheumatology DOF UK 102.
- Baeten D et al. N Engl J Med. 2015;373:2534–48.
- Marzo-Ortega H et al. Lancet Rheumatology. 2020;2:e339–46.
- Deodhar A et al. Arthritis Rheumatol. 2020 (epub ahead of print).
- Reich K et al. Br J Dermatol. 2019;181:954–66.
- Thaci D et al. J Am Acad Dermatol. 2015;73:400–9.
- Baraliakos X et al. Ann Rheum Dis. 2019;78. Abstract OP0235.
- McInnes IB et al. Lancet Rheumatol. 2020;2(4):e227-e235
- Baraliakos X et al. RMD Open. 2019;5(2):e001005.
- Braun J et al. Rheumatology (Oxford). 2019;58:859–68.
- Deodhar A et al. Ann Rheum Dis. 2020;79:722.
- Langley RG et al. N Engl J Med. 2014;371:326–38.
- McInnes IB et al. Lancet 2020;395:1496–505.
- Yiu Z et al. Br J Dermatol. 2020;183:294–302.
- Novartis Data on File. Secukinumab Cumulative Patient Numbers, Sec008. Feb 2023
- ClinicalTrials.gov. A study comparing AIN457 to placebo in subjects with a diagnosis of moderate to severe stable plaque psoriasis. https://clinicaltrials.gov/ct2/show/NCT00669916. Accessed 20 March 2023.
- Novartis Cosentyx® positive 16-week PREVENT results advance potential new indication for patients with axial spondyloarthritis [press release]. Basel, Switzerland. 17 September 2019. Novartis Cosentyx® positive 16-week PREVENT results advance potential new indication for patients with axial spondyloarthritis | Novartis Accessed 23 March 2023
- Novartis Q1 2022 Earnings. Available at: Q1 2022 Results (novartis.com) Accessed April 2022.



 *
*

